Physicochemical Properties of Alkyl Polyglycosides-Phase behavior
Binary systems
The phase diagram of the C12-14 alkyl polyglycoside (C12-14 APG)/ water system differs from that of the short-chain APG. (Figure 3). At lower temperatures, a solid/liquid region below the Krafft point is formed, it over a wide concentration range. With an increase in temperature, the system changes into an isotropic liquid phase. Because the crystallization is kinetically retarded to a considerable extent, this phase boundary changes position with the storage time. At low concentrations, isotropic liquid phase changes above 35℃ into a two-phase region of two liquid phases, as is normally observed with nonionic surfactants. At concentrations above 60% by weight, a sequence of liquid crystalline phase is formed at all temperatures. It is worth mentioning that in the isotropic single phase region, the obvious flow birefringence can be observed when the concentration is just lower than the dissolved phase, and then disappears rapidly after the shear process is completed. However, no polyphase region was found to be separated from the L1 phase. In the L1 phase, another region with weak flow birefringence is located near the minimum value of the liquid/liquid miscibility gap. 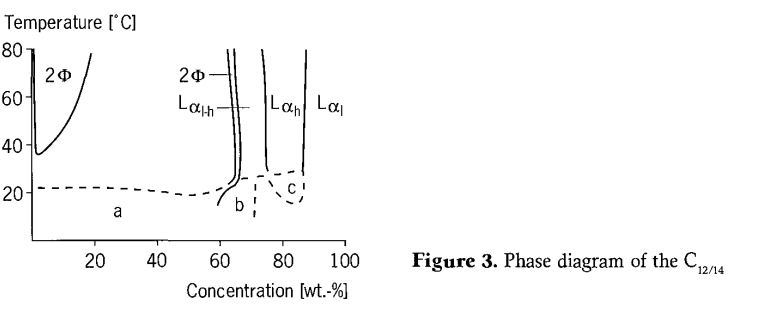
Phenomenological investigations into the structure of the liquid crystalline phases were conducted by Platz et al. Using such methods as polarization microscopy. Following these investigations, three different lamellar regions are considered in concentrated C12-14 APG solutions: Lαl , Lαl-h and Lαh. There are three different textures according to polarization microscopy.
After being stored for a long time, a typical lamellar liquid crystalline phase develops dark pseudoisotropic regions under polarized light. These regions are clearly separated from the highly birefringent areas. The Lαh phase, which occurs in the medium concentration range of the liquid crystalline phase region, at relatively high temperatures, shows such textures. Schlieren textures are never observed, although strongly birefringent oily streaks are usually present. If a sample containing an Lαh phase is cooled to determine the Krafft point, the texture changes below a characteristic temperature. The pseudoisotropic regions and the clearly defined oily streaks disappear. Initially, no C12-14 APG crystallizes, instead, a new lyotropic phase showing only weak birefringence is formed. At relatively high concentrations, this phase expands up to high temperatures. In the case of alkyl glycosides, a different situation emerges.All electrolytes, with the exception of sodium hydroxide, resulted in a significant reduction in cloud points.The concentration range of electrolytes is about an order of magnitude lower than that of alkyl polyethylene glycol ethers.Surprisingly, there are only very slight differences between individual electrolytes.The addition of alkali significantly reduced the cloudiness. To explain the behavioral differences between alkyl polyglycol ethers and alkyl polyglycol ethers, it is assumed that the OH group accumulated in the glucose unit has undergone different types of hydration with the ethylene oxide group. The significantly greater effect of electrolytes on the Alkyl polyglycol ethers suggests that there is a charge on the surface of the alkyl polyglycoside micelles, while the alkyl polyethylene glycol ethers assume no charge.
Thus, alkyl polyglycosides behave like mixtures of alkyl polyglycol ethers and anionic surfactants.The study of the interaction between alkyl glycosides and anionic or cationic surfactants and the determination of potential in the emulsion show that the alkyl glycosides micelles have a surface negative charge in the pH range of 3 ~ 9.In contrast, the charge of alkyl polyethylene glycol ether micelles is weakly positive or close to zero. The reason why alkyl glycoside micelles are negatively charged has not been fully explained.
Post time: Oct-22-2020





