The design requirements of an alkyl glycoside production plant based on Fisher synthesis depend largely on the type of carbohydrate used and the chain length of the alcohol used.The production of water-soluble alkyl glycosides based on octanol/decanol and dodecanol/tetradecanol was first introduced. Alkyl polyglycosides which, for a given DP, are insoluble in water on account of the alcohol used(number of C atoms in the alkyl chian≥16) are dealt with separately.
Under the condition of alkyl polyglucoside synthesis catalyzed by acid, secondary products such as polyglucose ether and colored impurities are generated.Polyglucose is an amorphous substance formed by glycosyl polymerization during the synthesis process.The type and concentration of the secondary reaction depends on the process parameters, such as temperature, pressure, reaction time, catalyst, etc.One of the problems solved by the development of industrial alkyl polyglycosides production in recent years is to minimize the formation of secondary products related to synthesis.
In general, short-chain alcohol-based (C8/10-OH)and low DP(large alcohol overdose) alkyl glycosides have the least production problems. In the reaction phase, with the increase of excess alcohol, the production of secondary products decreases. It reduces the thermal stress and removes excess alcohol during the formation of pyrolysis products.
Fisher glycosidation can be described as a process in which glucose reacts relatively quickly in the first step and oligomer equilibrium is achieved.This step is followed by a slow degradation of alkyl glycosides.The degradation process involves steps such as dealkylation and polymerization, which, at increased concentrations, irreversibly forms a thermodynamically more stable polyglucose.The reaction mixture exceeding the optimal reaction time is called overreaction.If the reaction is terminated prematurely, the resulting reaction mixture contains large amounts of residual glucose.
The loss of active substances of alkyl glucoside in the reaction mixture has a good relationship with the formation of polyglucose. In the case of excessive reaction, the reaction mixture gradually becomes polyphase again through the precipitation of polyglucose.Therefore, product quality and product yield are seriously affected by the time of reaction termination.Starting with solid glucose, the alkyl glycosides in the secondary products are lower in content, allowing the other polar components (polyglucose) and the remaining carbohydrates to be filtered out of the reactive mixture that has never fully reacted.
In the optimized process, the etherification product concentration is relatively low (depending on the reaction temperature, time, type of catalyst and concentration, etc.).
Figure 4 shows the typical course of a direct reaction of dextrose and fatty alcohol (C12/14-OH).
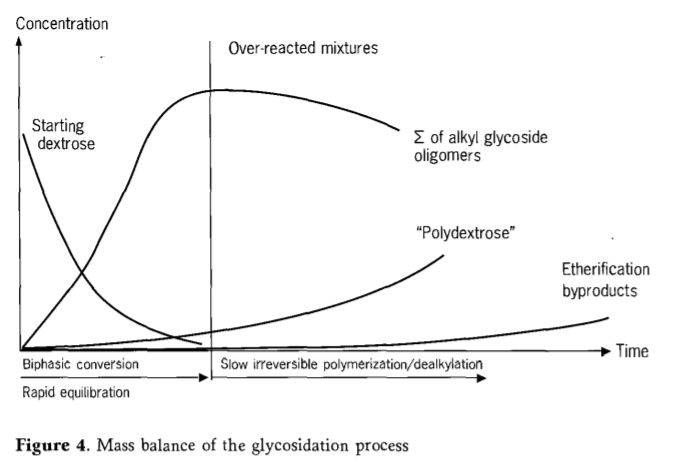
The temperature and pressure of the reaction parameters are closely related to each other in the fischer glycation reaction.In order to produce alkyl polyglycosides with low secondary products, pressure and temperature must be adapted to each other and strictly controlled.
Alkyl polyglycosides low in secondary products caused by low reaction temperatures (<100℃) in the acetalization. However, low temperatures result in relatively long reaction times(depending on the chain length of the alcohol) and low specific reactor efficiencies. Relatively high reaction temperatures(>100℃,typically 110-120℃) can lead to changes in color of the carbohydrates. By removing the lower-boiling reaction products (water in the direct synthesis,short-chain alcohols in the transacetalization process) from the reaction mixture, the acetalization equilibrium is shifted to the product side. If a relatively large amount of water is produced per unit of time, for example by high reaction temperatures, provision has to be made for the effective removal of this water from the reaction mixture. This minimizes secondary reactions(particularly the formation of polydextrose) which take place in the presence of water. The evaporation efficiency of a reaction stage depends not only on pressure, but also evaporation area,etc. Typical reaction pressures in the transacetalization and direct synthesis variants are between 20 and 100mbar.
Another important optimization factor is the development of selective catalysts in the glycosidation process, thus inhibiting, for example, polyglucose formation and etherification.As already mentioned, acetal or reverse acetal in Fischer synthesis is catalyzed by acids.In principle, any acid of sufficient strength is suitable for this purpose, such as sulfuric acid, p-toluene and alkyl benzenesulfonic acid and sulfonic succinic acid.The reaction rate depends on the acidity and the concentration of the acid in the alcohol.Secondary reactions that can also be catalyzed by acids (e.g., polyglucose formation) occur primarily in the polar phase (trace water) of the reaction mixture, and alkyl chains that can be reduced by the use of hydrophobic acids (e.g., alkyl benzenesulfonic acid) are dissolved primarily in the less polar phase of the reaction mixture.
After the reaction, the acid catalyst is neutralized with an appropriate base, such as sodium hydroxide and magnesium oxide.The neutralized reaction mixture is a pale yellow solution containing 50 to 80 percent fatty alcohols. High fatty alcohol content is due to the molar ratio of carbohydrates to fatty alcohols. This ratio is adjusted to obtain a specific DP for industrial alkyl polyglycosides, and is usually between 1:2 and 1:6.
The excess fatty alcohol is removed by vacuum distillation. Important boundary conditions include:
– Residual fatty alcohol content in the product must be <1% because other
wise solubility and odor are adversely affected.
- To minimize the formation of unwanted pyrolysis products or discoloring components, thermal stressing and residence time of the target product must be kept as low as possible in dependence upon the chain length of the alcohol.
- No monoglycoside should enter the distillate because the distillate is re- cycled in the reaction as pure fatty alcohol.
In the case of dodecanol/tetradecanol, these requirements are used for the removal of excess fatty alcohols, which are largely satisfactory through multistage ditillation. It is important to note that as the content of fatty alcohols decreases, the viscosity increases significantly. This obviously impairs heat and mass transfer in the final distillation phase.
Therefore, thin or short -range evaporators are preferred. In these evaporators, the mechanically moving film provides higher than evaporation efficiency and shorter product residence time, as well as good vacuum. The final product after distillation is an almost pure alkyl polyglycoside, which accumulates as a solid with a melting point of 70℃ to 150℃. The main process steps of alkyl synthesis are summarized as Figure 5.
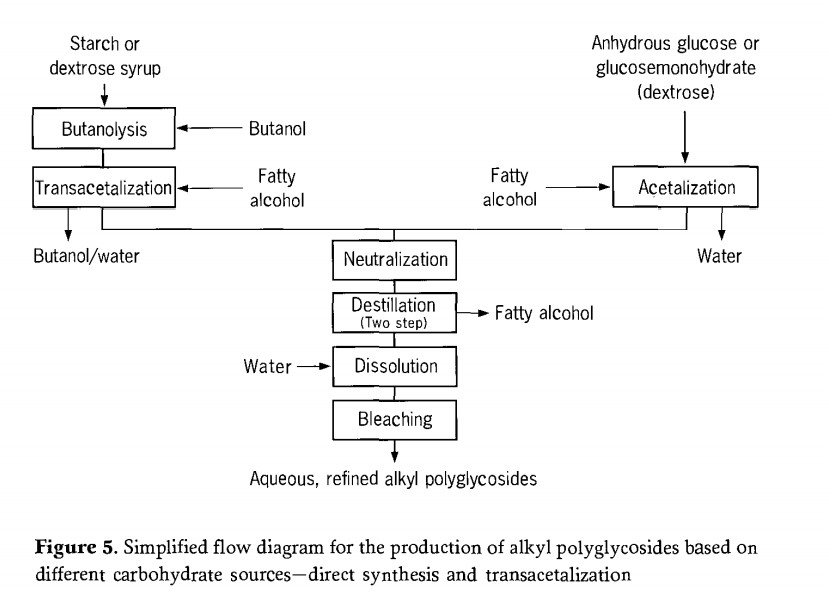
Depending on the manufacturing process used, one or two alcohol cycle flows accumulate in the production of alkyl polyglycoside; excess fatty alcohols, whereas short-chain alcohols can be almost completely recovered. These alcohols may be reused in subsequent reactions. The need for purifying or the frequency with which purifying steps have to be carried out depends upon the impurities accumulated in the alcohol. This is largely dependent upon the quality of the preceding process steps (for example reaction, alcohol removal).
After removal of the fatty alcohol, the alkyl polyglycoside active substance is directly dissolved in water so that a highly viscous 50 to 70 % alkyl polyglycoside paste is formed. In subsequent refining steps, this paste is worked up into a product of satisfactory quality in accordance with performance-related requirements. These refining steps may comprise bleaching of the product, the adjustment of product characteristics, such as Ph value and active substance content, and microbial stabilization. In the patent literature, there are many examples of reductive and oxidative bleaching and two-stage processes of oxidative bleaching and reductive stabilization. The effort and hence the cost involved in these process steps to obtain certain quality features, such as color, depend on performance requirements, on the starting materials, the DP required and the quality of the process steps.
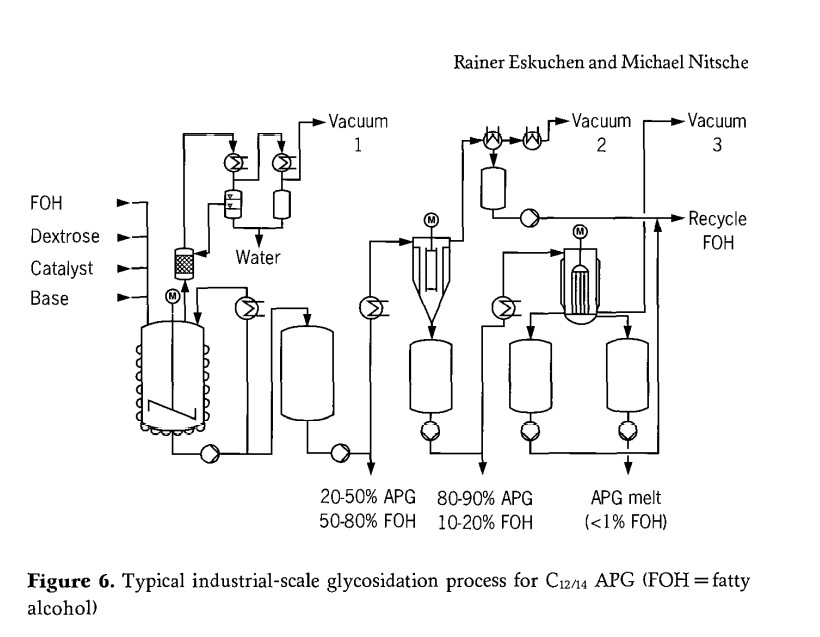
Figure 6 illustrates an industrial production process for long-chain alkyl polyglycosides ( C12/14 APG) via direct synthesis)
Post time: Oct-13-2020





