Synthesis of alkyl polyglycoside butyl ethers
A frequently required property of alkyl polyglycosides is enhanced foamability. However, in many applications, this feature is actually considered disadvantageous. Therefore, there is also interest in developing alkyl polyglycoside derivatives that combine good cleaning performance with only a slight tendency to foam. Considering this goal, alkyl polyglycoside butyl ether was synthesized. It is known in the literature that alkyl glycosides can be capped with alkyl halides or dimethyl sulfate in alkaline aqueous solutions.
On an industrial scale, the reaction in aqueous solution is a disadvantage because concentrated water-free products cannot be obtained without additional working-up steps. Therefore, a water-free process was developed, which is outlined in Figure 6. The alkyl polyglycoside is initially introduced into the reactor with an excess of butyl chloride and heated to 80℃. The reaction is initiated by addition of potassium hydroxide as the catalyst. On completion of the reaction, the reaction mixture is neutralized, the potassium chloride precipitate is filtered off and the excess butyl chloride is distilled off. The product is composed of various alkyl polyglycosides and alkyl polyglycoside butyl ethers. According to GC analysis, the ratio of alkyl monoglycoside, alkyl mono-glycoside monobutyl ether and alkyl monoglycoside polybutyl ether is 1:3:1.5.
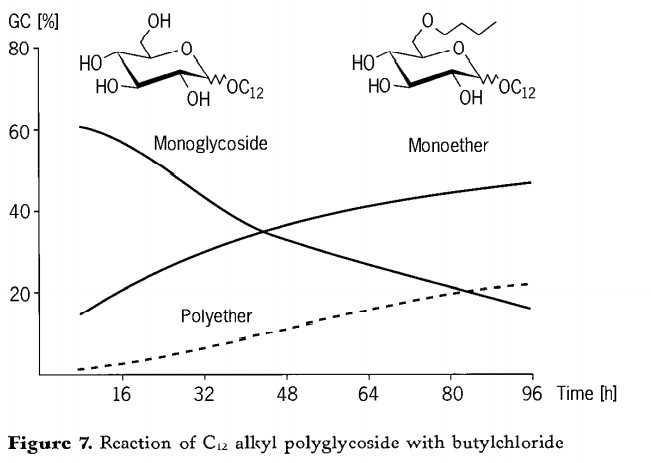
The course of the reaction for the etherification of a C12 alkyl polyglycoside is shown in Figure 7. The monoglycoside content decreases from around 70% to less than 20%. At the same time, the value for the monoether rises to 50%. The more monobutyl ether present, the more polybutyl ethers can be formed therefrom. Only after 24hours is there any significant formation of polybutyl ethers. As expected, the content of polyethers increases with increasing reaction time. However, a value of 20% is not exceeded. The average etherification degree is 1 ~ 3 butyl per alkyl glycoside unit. The reaction effect of C12 alkyl glycoside was the best. In the case of N =8 or 16 alkyl polyglycoside butyl ether, the results deteriorated.
From these three examples it is clear that derivatives of alkyl glycosides are readily available.The special uses envisaged also depend on the surface-activity properties of these derivatives.
Post time: Apr-09-2021