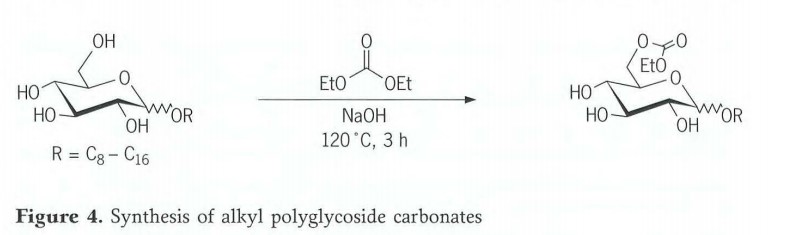
Synthesis of Alkyl polyglycoside carbonates
Alkyl polyglycoside carbonates were prepared by transesterification of alkyl monoglycosides with diethyl carbonate (Figure 4). In the interests of thorough mixing of the reactants, it has proved to be of advantage to use the diethyl carbonate in excess so that it serves both as transesterification component and as solvent. 2Mole-% of a 50% sodium hydroxide solution are added dropwise to this mixture with stirring at around 120℃.After 3hours under reflux, the reaction mixture is allowed to cool to 80℃ and neutralized with 85% phosphoric acid. The excess diethyl carbonate is distilled off in vacuo. Under these reaction conditions, one hydroxyl group is preferably esterified. The ratio of remaining educt to products in 1:2.5:1(monoglycoside: Monocarbonate:Polycarbonate).
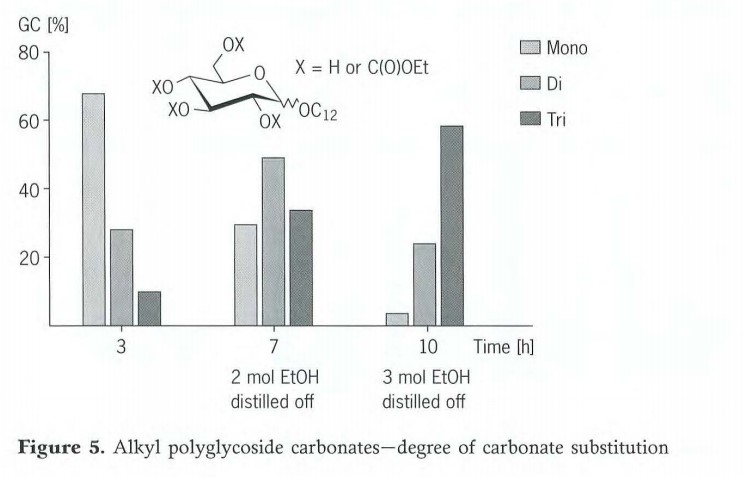
Besides the monocarbonate, products with a relatively high degree of substitution are also formed in this reaction. The degree of carbonate addition can be controlled by skilled management of the reaction. For a C12 monoglycoside, a distribution of mono-,di- and tricarbonate of 7:3:1 is obtained under the reaction conditions just described (Figure 5). If the reaction time is increased to 7hours and if 2moles of ethanol are distilled off in that time, the main product is C12 monoglycoside dicarbonate. If it is increased to 10hours and 3moles of ethanol are distilled off, the main product ultimately obtained is the tricarbonate. The degree of carbonate addition and hence the hydrophilic/lipophilic balance of the alkyl polyglycoside compound can thus be conveniently adjusted by variation of the reaction time and the distillate volume.
Post time: Mar-22-2021